On Wednesday, May 27, Zynerba (NASDAQ:ZYNE) reported positive data from its phase II trial testing its cannabinoid drug Zygel to treat pediatric and adolescent patients with autism spectrum disorder (ASD). The stock rose in Wednesday morning trading to $6.30 per share before settling back to close Wednesday at $5.57 per share.
After the market closed on May 8, SanaCurrents added the ASD indication as a catalyst for Zynerba. The company’s stock traded at $3.99 per share at the time, providing a 39.5% return for the ASD data in just a few weeks.
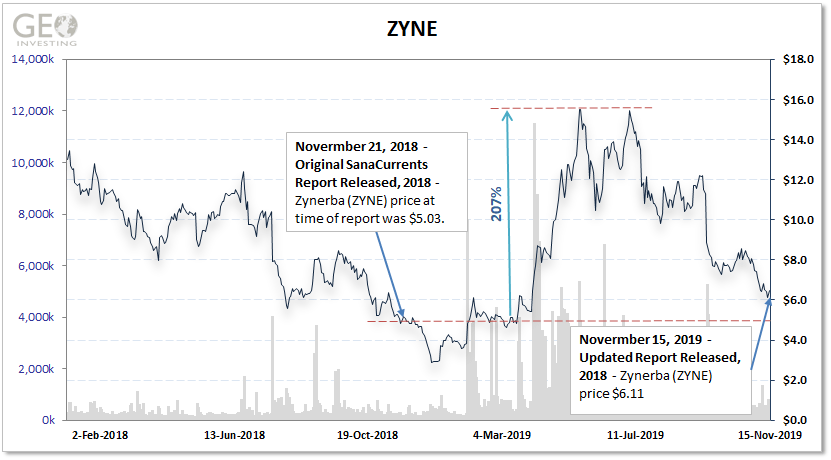
SanaCurrents first issued a report on Zynerba’s CONNECT-FX phase II trial in November 2018 when the stock traded at $5.03 per share. In the CONNECT-FX trial, Zynerba is testing Zygel to treat 3- to 17-year-old autistic patients with Fragile X syndrome, a rare genetic developmental disability.
SanaCurrents updated the new June 2020 date for the Fragile X data release in November 2019.
Between these 2 CONNECT-FX report dates, there was a notable 200% climb in ZYNE’s stock price, likely due to investors’ anticipation of the data stated to be released in Q3, 2019, which never happened, and has still yet to happen.
The CONNECT-FX trail includes 212 patients, at clinical sites around the world. It also is a randomized, double blind, placebo-controlled trial, indicating the efficacy and responses in the Zygel arm will be compared to patients who received a placebo.
The CONNECT-FX trial remains more important for Zynerba as the company claims it will seek an NDA for Zygel. However, Zynerba has delayed reporting the CONNECT-FX results for nine months. In the interim, the company’s stock shot up during 2019 to more than $16 per share based on a buoyant market for cannabidiol (CBD) treatments such as Zygel.
In contrast, the phase II trial in ASD involved 37 patients at a single clinical site, testing Zygel over 14 weeks in an open label trial. Though the results were strong, there was not a comparison to a placebo arm in the ASD trial.
The ASD data proved to be a brief, small catalyst for the company’s stock.
With the ASD data catalyst now complete, SanaCurrents is suspending its sentiment on the CONNECT-FX trial and placing capital currently invested in Zynerba into future, pending SanaCurrents catalysts
As seen last week from late-breaking trial results provided by Harpoon Therapeutics (NASDAQ:HARP), data readouts can be positive but underwhelming. Given Zynerba’s delay in reporting the results from the larger Fragile X trial, the CONNECT-FX data likely could be similar to Harpoon’s – respectable but not compelling.
If the CONNECT-FX data does prove to be a setback for Zynerba, the company still will be able to claim it is advancing Zygel for the larger patient indication, ASD.
Disclosure: Suspending coverage on ZYNE and closing position; placing capital in future pending catalysts.